What is DIREct?
Although treatment of non-small cell lung cancer (NSCLC) with immune checkpoint inhibitors (ICI) can produce remarkably durable responses, most patients develop early disease progression. Furthermore, initial response assessment by conventional imaging is often unable to identify which patients will achieve durable clinical benefit (DCB). Here, we demonstrate that pre-treatment circulating tumor DNA (ctDNA) and peripheral CD8 T cell levels are independently associated with DCB. We further show that ctDNA dynamics after a single infusion can aid in identification of patients who will achieve DCB. Integrating these determinants, we developed and validated an entirely noninvasive multi-analyte assay (DIREct- On, Durable Immunotherapy Response Estimation by immune profiling and ctDNA- On-treatment) that robustly predicts which patients will achieve DCB with higher accuracy than any individual feature. Taken together, these results demonstrate that integrated ctDNA and circulating immune cell profiling can provide accurate, noninvasive, and early forecasting of ultimate outcomes for NSCLC patients receiving PD-(L)1 blockade-based ICI.
What parameters are needed for DIREct response prediction?
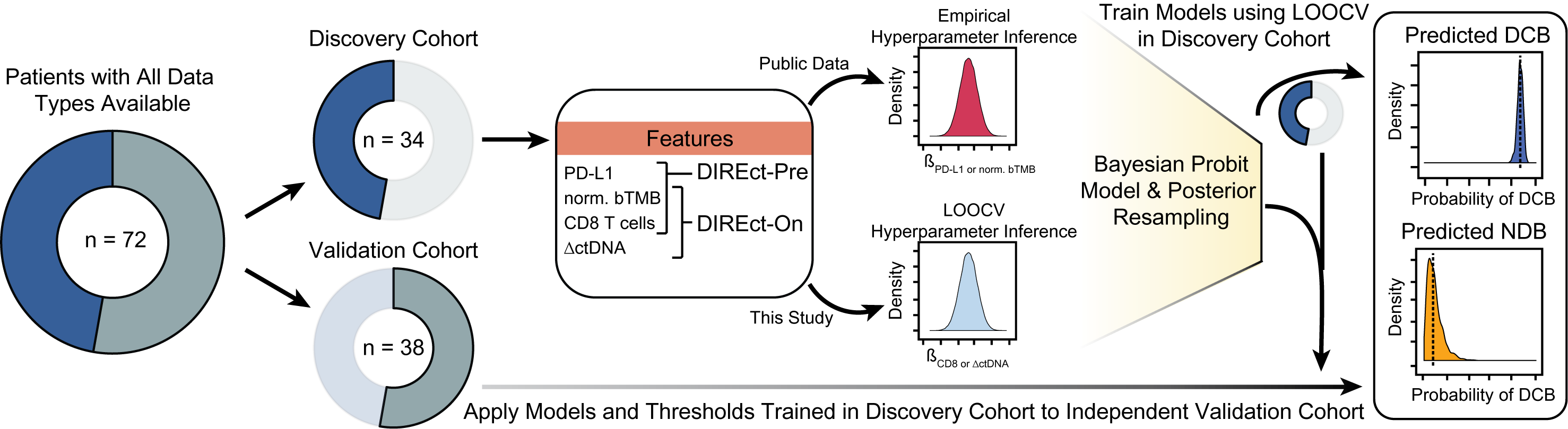
The DIREct-On model incorporates three elements, ctDNA normalized (blood) TMB, CD8 fractions, and ctDNA fold change from pre to on-treatment (within 4 weeks).
How does the model work?
The DIRect model is a Bayesian model where prior probabilities are generated empirically, using either publicly available data or the data generated in Nabet, Esfahani, et al. The model creates a score for each patient along with a credible interval summarizing the uncertainity around the predicted score. This score is used as the indicator of patient's response to ICI.
Can one improve or update the DIREct model using new data?
Yes. The DIREct model is a Bayesian framework which inherently is capable of being updated (i.e. updating priors to posteriors). The website version, however, is a pre-trained model. For updating the model, the user needs to download the source code and make the changes on that.
Please send questions, issues, and/or licensing requests to: directon.model@gmail.com